Background: Adverse events (AEs) such as CRS and NEs of varying severity have been associated with CAR T cell therapies, including liso-cel, an autologous, CD19-directed, 4-1BB CAR T cell product administered at equal target doses of CD8 + and CD4 + CAR + T cells. Liso-cel has previously demonstrated clinically meaningful responses in patients with R/R FL in the phase 2 TRANSCEND FL study (NCT04245839). In addition, previous research among patients with large B-cell lymphoma has suggested an acceptable safety profile with few grade ≥ 3 CRS/NEs, and safety data from TRANSCEND FL are in line with this larger dataset. However, there is limited to no research published on estimates of HCRU and costs related to the management of CRS/NEs among liso-cel-treated patients with FL. This analysis evaluated HCRU and estimated costs of managing CRS/NEs by grade and concurrency for patients with R/R FL who received liso-cel treatment in the TRANSCEND FL study.
Methods: Retrospective analyses were conducted for liso-cel-treated patients with R/R FL (second line or later) who experienced CRS/NEs within 90 days after infusion using patient-level HCRU data from the TRANSCEND FL study databases. HCRU captured included facility (standard inpatient [IP] and ICU hospitalizations), length of stay (LOS), diagnostics (eg, laboratory and imaging), procedures (eg, dialysis and mechanical ventilation), and medications (eg, oncology supportive care, prophylactics, and other AE management). Costs of managing CRS/NEs were estimated using a microcosting methodology in which 1) HCRU from the time of AE onset through resolution was identified and 2) the most recently available unit costs sourced from public databases or literature (US Centers for Medicare and Medicaid Services, Healthcare Cost and Utilization Project, and IBM ® Micromedex ® RED BOOK ®) were applied to each HCRU and adjusted to 2023 US dollars using the Consumer Price Index. Analyses were stratified by AE grade and concurrency of events (CRS or NEs only, nonconcurrent CRS and NEs, and concurrent CRS and NEs). Patients with nonconcurrent and concurrent CRS/NEs were categorized by the highest-grade event experienced.
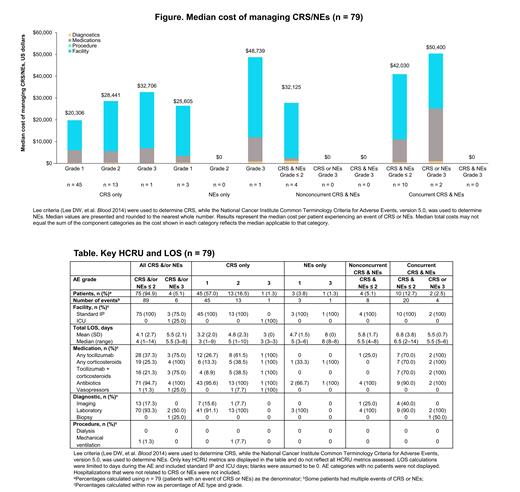
Results: CRS only occurred in 59/130 (45%) patients, NEs only occurred in 4/130 (3%), and CRS and/or NEs occurred in 79/130 (61%). Of the 79 patients who experienced CRS and/or NEs, 75 (95%) were grade ≤ 2 and 4 (5%) were grade 3; no grade 4 or 5 CRS/NEs were observed. Most CRS/NEs were experienced as a single event (63/79 [80%]). Patients who experienced CRS and/or NEs had a median (range) age of 60 (23‒78) years and were predominantly male (51/79 [65%]). Estimated median management costs ranged from $20,306 (grade 1 CRS only) to $50,400 (grade 3 CRS or NEs; Figure). The largest contributor to median management costs across CRS/NE categories was facility HCRU. Key HCRU and total LOS (standard IP hospitalization and ICU stay days among hospitalized patients) are shown in the Table. Almost all patients (78/79 [99%]) had a standard IP hospitalization, and only 1 patient (1%) with a grade 3 CRS only event was admitted to the ICU. Median (range) total LOS for IP/ICU admission was 1.5 days longer among patients with grade 3 versus grade ≤ 2 CRS and/or NEs (5.5 [3‒8] days vs 4 [1‒14] days). Although there were very few grade 3 events (n = 4) to assess, patients with grade 3 versus grade ≤ 2 CRS and/or NEs also had higher rates of utilization for some medications (eg, tocilizumab: 3/4 [75%] vs 28/75 [37%]; corticosteroids: 4/4 [100%] vs 19/75 [25%]).
Conclusions: While most patients with R/R FL treated with liso-cel in the TRANSCEND FL study experienced treatment-emergent CRS and/or NEs, they were almost all of low severity (grade ≤ 2) and experienced as single events. Management costs for CRS/NEs, primarily related to facility HCRU, increased with greater AE severity and concurrency. The additional median cost for a patient with grade 3 versus grade ≤ 2 CRS or NEs was $22,586, representing an 88% increase in cost; however, this finding must be considered in the context of the small number of overall grade 3 events (n = 4) and ICU admissions (n = 1). CAR T cell therapies with few associated higher severity AEs provide a potential option to optimize resource use and costs for the management of CRS/NEs in patients with FL.
Disclosures
Saunders:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Singh:Bristol Myers Squibb: Research Funding. Lee:Bristol Myers Squibb: Research Funding. Farazi:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Fasan:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company; Sanofi Genzyme: Speakers Bureau; Oncopeptides: Other: Advisory Board, Speakers Bureau. Mirza:Bristol Myers Squibb: Consultancy; BluePath Solutions: Current Employment. McGarvey:Bristol Myers Squibb: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal